
The two different coloured Co(II) complex ions, 2+ and 2-, exist together in equilibrium in solution in the presence of chloride ions:
#COBALT CHLORIDE LE CHATELIER LAB HOW TO#
I have indeed had my students use some of these experiments as part of a laboratory-based exercise, and on other occasions I have used these experiments as in-class demonstrations.ĭo you have any ideas for how to extend or modify the experiments presented here? If so, I’d love to hear any suggestions you might have.ġ. On the other hand, addition of silver nitrate causes the removal of Cl - ion because silver ions precipitate out AgCl (s):īecause removal of Cl - ion has the opposite effect of Cl - addition, addition of silver nitrate shifts each equilibrium listed in Scheme I to the left, ultimately resulting in a color change to blue.īecause these experiments avoid the use of concentrated HCl, they are amenable to having students perform these copper-based equilibrium experiments as part of a laboratory-based exercise. The presence of high Cl - concentration causes each reaction to shift to the right, ultimately forming CuCl 4 2- (Scheme I). However, you will also note in the video above that addition of salt to this system causes a shift to an orange color, and addition of silver nitrate causes a shift to a blue color. Using this system of Cu 2+ complexes, I have only been able to generate colors that range from green to yellow by varying temperature. Video 2: Chemical Equilibrium: Colorful Demonstrations , Tommy Technetium YouTube Channel (accessed ) Indeed, I have noted that solutions of CuCl 2 in acetone are dirty-yellow at room temperature, green when incubated in a dry-ice acetone mixture, and orange-yellow when incubated in hot water (Video 2). Because of this, each reaction shifts to the right at increasing temperatures, but to the left at decreasing temperatures. Note that each reaction listed in Scheme I is endothermic from left to right. Each reaction is endothermic from left to right. Scheme I: Putative compounds, reactions, and colors involved in the formation of various copper-chloro complexes in acetone. 4 This system of Cu 2+ complexes has the added benefit of displaying a larger variety of colors: blue, green, yellow, and orange (Scheme I). 1-3 Because concentrated HCl presents several hazards, I have instead begun using a system of Cu 2+ complexes, which does not require the use of concentrated acid, to explore chemical equilibrium. In this demonstration, concentrated HCl (12 M) is used as the source of Cl - to produce the blue colored CoCl 4 2- species.

Video 1: Cobalt equilibrium and thermodynamics, Tommy Technetium YouTube Channel (accessed ) Therefore, the above reaction can be shifted to the right at high temperatures or to the left at low temperatures (Video 1). The reaction that forms CoCl 4 2- is endothermic. When Co 2+ is dissolved in water it appears pink, but it forms a blue-colored complex in the presence of high concentrations of chloride ion: 1-3Ĭo 2+(aq) + 4 Cl -(aq) ←→ CoCl 4 2-(aq) Equation 1
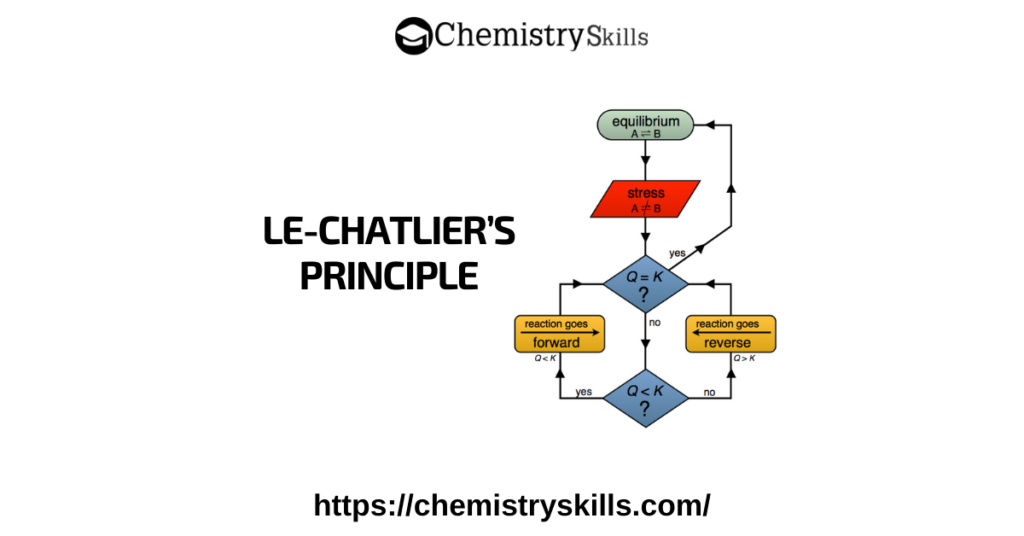

A popular method for demonstrating the effect of temperature on chemical equilibrium involves the use of Co 2+ complexes.


 0 kommentar(er)
0 kommentar(er)
